Designed For Purity, XEOMIN Has Only The Ingredients It Needs To Deliver Results
What is Cervical Dystonia?
Cervical dystonia is a movement disorder causing involuntary muscle contractions in the neck that may twist and turn your head into uncomfortable positions. Symptoms may include painful muscle spasms, a tilted or rotated head, and the frustration of not being able to control your own movements.
Examples of Abnormal Postures

Head tilts forward

Head tilts backwards

Head rotates to left or
right side

Head tilts to the side
Images are for illustration purposes only. Individual results may vary.
* Cervical dystonia is the most common form of focal dystonia
* Estimated to affect more than 60,000 people in the United States in 20231
What is XEOMIN?
XEOMIN is an FDA-approved prescription medication that is used to treat adult upper limb spasticity
XEOMIN is a botulinum toxin type A, which may help decrease muscle contractions and neck pain.2
XEOMIN is injected by your doctor

It became more painful to me, and obvious to others who pointed out that my head was tilted. It’s frustrating losing control of my own body.
45 year old
Not an actual patient.
How Does XEOMIN Work?
XEOMIN works by blocking the signals your nerves send to the muscles in your arm, so that these muscles can relax and not be as tight. This can improve your ability to move those muscles.
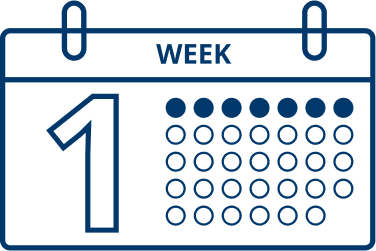
Patients typically start to see results one week after they start treatment with XEOMIN.
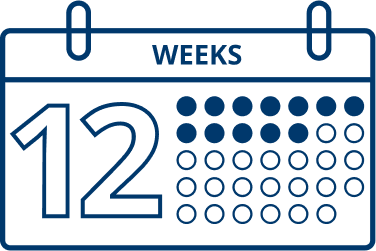
In clinical trials, most patients were retreated after 12 to 14 weeks.
Where is XEOMIN Injected?


Where Is
XEOMIN
Injected?
Your doctor will locate certain areas of the neck and shoulders and inject XEOMIN directly into those areas. If needed, retreatment with XEOMIN can occur every 12 weeks.
You and your doctor can decide the right plan for you.
Possible Muscles Involved

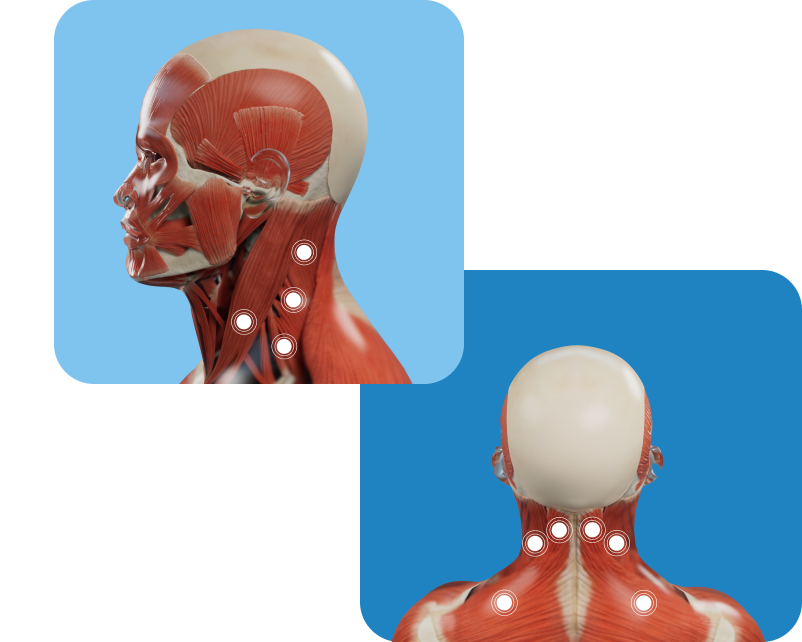
Markings show examples of muscles that may be treated for cervical dystonia in adults; however, not all possible injection locations are shown. Not all patients will receive treatment in the same muscle(s). Your doctor will determine the appropriate locations and doses for XEOMIN injection.

Not an actual patient.
How Can XEOMIN Help You?
In clinical studies, XEOMIN was shown to reduce symptoms associated with adult cervical dystonia, including abnormal neck position and neck pain.

48%
(322/669)
of patients experienced a meaningful decrease in pain† after their first XEOMIN injection
†Greater than 30% pain reduction = meaningful decrease in pain.

10%
(69/669)
of patients experienced complete pain relief after their first treatment with XEOMIN§
§The analyses in this study were based on patients who had a baseline pain assessment and reported experiencing pain at baseline. Because several studies with different designs were combined for this analysis, it was not possible to compare pain responses of patients receiving XEOMIN with those patients receiving placebo or a different comparator drug in their first injection cycle. Individual results may vary.
Designed For Purity, XEOMIN Has Only The Ingredients It Needs To Deliver Results
Designed For Purity, XEOMIN Has Only The Ingredients It Needs To Deliver Results

XTRACT Technology is a state-of-the-art manufacturing process that removes the unnecessary proteins.
XEOMIN is uniquely purified to contain only the therapeutic component. It utilizes XTRACT Technology®, a state-of-the-art manufacturing process that removes unnecessary proteins, and makes XEOMIN different from other treatments.*
Repeated exposure to neurotoxins with complexing proteins may cause a person to develop antibodies to treatment, which may result in the treatments not working as well as they once did.†
is a state-of-the-art manufacturing process that removes the unnecessary proteins
The most common side effects in clinical trials of XEOMIN in adults with cervical dystonia were:
Difficulty Swallowing|
Neck Pain|
Muscle Weakness
Pain at the Injection Site|
Muscle and Bone Pain
This information is not meant to imply superiority of safety or efficacy of any toxin.
†It’s possible to develop antibodies with all botulinum toxins, including XEOMIN.

Move Forward with XEOMIN, a Uniquely Purified Option for Cervical Dystonia
XEOMIN has been proven safe and effective and used in 6.5 million patients from more than 75 countries, for more than 12 years.
Not an actual patient. Individual results may vary.
Move Forward with XEOMIN, a Uniquely Purified Option for Cervical Dystonia
XEOMIN has been proven safe and effective and used in 6.5 million patients from more than 75 countries, for more than 12 years.
Not an actual patient. Individual results may vary.
Support to Help You Start and Stay on Xeomin

Patient
Savings Program

Visit MERZCONNECT.com for additional information to help you get started and stay on XEOMIN.
XEOMIN Patient Savings is Available in Just 3 Easy Steps.
Enroll in the program*
Receive XEOMIN treatment
Obtain program savings*†
*Restrictions apply to eligibility. Commercial insurance required.
Reimbursement limited to out-of-pocket XEOMIN medication costs and related administration fees. State limitations may apply. Please see Full Terms and Conditions at MERZCONNECT.com. Merz reserves the right to change XEOMIN Patient Savings Program Terms and Conditions, including the eligibility requirements, at any time. This is not health insurance.
†You may be required to pay upfront for your co-pay/co-insurance, as determined by your insurance coverage/policy and your healthcare provider’s co-pay collection practice.
Once you and your doctor have decided XEOMIN is right for you, MERZ CONNECT offers you savings and support to help you get started and stay on therapy.

Reclaim Life’s Moments
with XEOMIN
If You’re New to Treatment, or Your Current or Past Therapy isn’t Working as Well As it Used to, Talk to Your Doctor Today!
Reclaim Life’s Moments
with XEOMIN
If You’re New to Treatment, or Your Current or Past Therapy isn’t Working as Well As it Used to, Talk to Your Doctor Today!
