Designed For Purity, XEOMIN Has Only The Ingredients It Needs To Deliver Results
Designed For Purity,
XEOMIN Has Only
The Ingredients
It
Needs To Deliver
Results
Chronic Sialorrhea in Pediatrics
XEOMIN for Chronic Sialorrhea
Xeomin Is the First and Only FDA-Approved Neuromodulator
For Pediatric Patients With Chronic Sialorrhea

The most common cause of sialorrhea is a nerve and muscle condition, but it can also be caused by problems with brain signaling, muscular problems, or excessive secretion of saliva.1
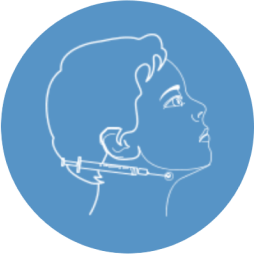
Xeomin blocks certain chemicals at the point where salivary glands and nerves meet. It slows down the release of the chemicals at the nerve endings that promote saliva production.2
Images are for illustration purposes only. Individual results may vary.
What is Chronic Sialorrhea?
What is Chronic Sialorrhea in Pediatrics?
- Sialorrhea is drooling or excessive salivation1
- It is normal in infants, but usually stops by 15-18 months of age3
Causes4
- Cerebral palsy (CP)
- Traumatic brain injury
- Epilepsy
- Congenital abnormality of brain development, such as Down syndrome, autism, Angelman syndrome, or Rett syndrome
Symptoms1
- Hypersalivation (excessive drooling)
- Skin breakdown around the mouth
- Infection from the skin breakdown
- Dehydration or not enough fluids
- Foul odor
445,000 children under the age of 18 are estimated to suffer from pediatric sialorrhea in the United States4,5
Impact on Daily Living6-12
Social6-9
- Embarrassment
- Social isolation
- Stigmatization
Medical10,11
- Breathing problems or clogged airways
- Skin irritation
- Swallowing or feeding difficulties
- Dehydration
XEOMIN Helps Improve Chronic Sialorrhea Symptoms
In clinical studies, pediatric patients had significant and sustained reduction in saliva over 16 weeks.2
Chronic Sialorrhea in Pediatrics
Xeomin Is the First and Only FDA-Approved Neuromodulator
For Pediatric Patients With Chronic Sialorrhea

The most common cause of sialorrhea is a nerve and muscle condition, but it can also be caused by problems with brain signaling, muscular problems, or excessive secretion of saliva.1

Xeomin blocks certain chemicals at the point where salivary glands and nerves meet. It slows down the release of the chemicals at the nerve endings that promote saliva production.2
Images are for illustration purposes only. Individual results may vary.
XEOMIN Helps Improve Chronic Sialorrhea Symptoms
In clinical studies, pediatric patients had significant and sustained reduction in
saliva over 16 weeks.2
What is Chronic Sialorrhea in Pediatrics?
- Sialorrhea is drooling or excessive salivation1
- It is normal in infants, but usually stops by 15-18 months of age2
Causes3
- Cerebral palsy (CP)
- Traumatic brain injury
- Epilepsy
- Congenital abnormality of brain development, such as Down syndrome, autism, Angelman syndrome, or Rett syndrome
Symptoms1
- Hypersalivation (excessive drooling)
- Skin breakdown around the mouth
- Infection from the skin breakdown
- Dehydration or not enough fluids
- Foul odor
445,000 children under the age of 18 are estimated to suffer from pediatric sialorrhea in the United States3,4
Impact on Daily Living6-11

Social5-8
- Embarrassment
- Social isolation
- Stigmatization
Medical9,10
- Breathing problems or clogged airways
- Skin irritation
- Swallowing or feeding difficulties
- Dehydration

Does this seem familiar?
Emma and Jacob* are children with chronic sialorrhea >
*Images and fictionalized stories are for illustration purposes only. Not actual patients.



